SARS-CoV-2 Antibody Detection and Quantification
Antibody response to SARS-CoV-2 is usually detectable 2-3 weeks after infection, although in some cases it may take longer. Antigen-specific IgA and IgM levels start to decline after a few weeks but IgG titres have been shown to decrease at a much slower rate. Combining the measurement of multiple antibody isotypes has been shown to improve the predictive accuracy of serological tests. Anti-RBD IgG concentrations have been shown to correlate with neutralising antibody titres.
Serological testing is a useful tool with a variety of potential applications:
- Retrospective contact tracing
- Identification of patients that have had asymptomatic infections
- Seroprevalence surveillance
- Assessment of herd immunity & longevity of protective immunity
- Assessment of vaccine efficacy
- Monitoring nAb titers in vaccinees after mass vaccination
Oxford Biosystems has several kits available for antibody detection.
Contact us for more information regarding our range of products detailed below for COVID-19 antibody detection, including sensitivity and specificity.
SD Biosensor Standard Q COVID-19 IgM/IgG PLUS is a lateral flow device that can be read by eye.
- Rapid results in 10-15 minutes
- Differentiation of IgG and IgM antibodies
- Whole blood (finger prick), serum or plasma samples
- CE marked
- Ready-to-use and room temperature storage
- No additional equipment or reagents necessary
SD Biosensor Standard F COVID-19 IgM/IgG Combo FIA is a rapid fluorescent immunoassay for use with the Standard F analysers.

- Rapid results in 15 minutes
- Differentiation of IgG and IgM antibodies
- Whole blood (finger prick), serum or plasma samples
- CE marked
- Ready-to-use and room temperature storage
- Automatic reading and analysis of results
- F2400 analyser provides random access testing for up to 60 samples per hour
- Data management via LIS/HIS connectivity
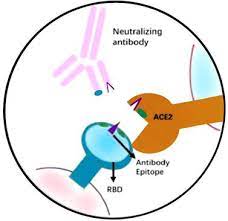
SD Biosensor Standard F SARS-CoV-2 nAb FIA is a rapid fluorescent immunoassay and a surrogate VNT (virus neutralisation test) for the detection of total immuno-dominant neutralising antibodies (nAb) targeting the spike protein RBD in serum and plasma.
A neutralising antibody is one that defends a cell from a virus by neutralising the biological effect. It prevents the virus from interacting with host cells by binding to the virus surface Ag.
- Rapid results in 35 minutes
- Serum or plasma samples
- Recombinant ACE2 & RBD proteins mimic the virus-host interaction
- Differentiates antibodies to SARS-Cov-2 variants
- Differentiates antibodies to SARS-Cov-2 variants
- Ready-to-use and room temperature storage
- Automatic reading and analysis of results on F200 or F2400 instruments
TestLine Clinical Diagnostics, a Czech company and part of the BioVendor Group, has developed a range of ELISA kits for the determination of specific IgG, IgM and IgA antibodies against SARS-CoV-2 (COVID-19) in human serum or plasma.
- ELISA kits optimized and validated for detection of IgA, IgG and IgM antibodies against recombinant SARS-CoV-2 Nucleocapsid protein (NP) or S1 Receptor-binding domain (RBD)
- ELISA kits for manual use and automated on a variety of ELISA processing platforms
- SmartEIA kits, specifically designed for automated analysis using the Dynex Agility ELISA instrument
- Microblot Array for the simultaneous detection of antibodies to multiple proteins and efficient multiplex diagnostics
For more information on the Testline product range for COVID-19 visit their website