Neutralising SARS-CoV-2 antibodies
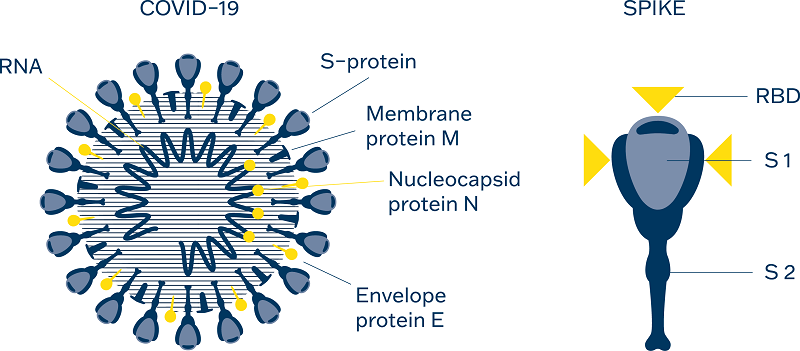
The Spike Protein of SARS-CoV-2 is a trimer made up of S1 and S2 proteins.
The Receptor Binding Domain (RBD) of S1 binds to the ACE2 receptor in the cell membrane to gain access to the host cell. Spike antigens trigger the host’s cellular immune system resulting in the B cells producing antibodies against Spike antigens.
There are many commercial kits available to detect antibodies to the SARS-CoV-2 virus, predominantly antibodies specifically against the nucleoprotein (NP) and/or the spike protein (SP) of the virus. But does a positive antibody test mean you have protective immunity?
Most tests do NOT distinguish between neutralising and non-neutralising antibodies!
Question: What is a Neutralising Antibody (nAb)?
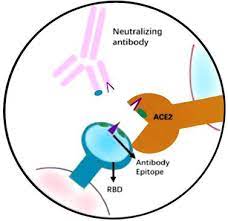
Answer: A Neutralising antibody is one that defends a cell from a virus by neutralising the biological effect of the virus. It prevents the virus from interacting with host cells by binding to the virus surface antigen. The majority of SARS-CoV-2 nAb are directed against the receptor-binding domain (RBD) of S protein. These antibodies block the binding of RBD and the ACE2 receptor.
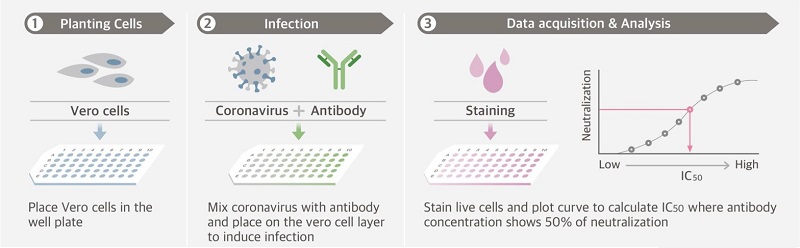
The conventional test for detecting neutralising antibodies is the virus neutralisation test (VNT). This test is performed by skilled laboratory staff in a BSL3 laboratory facility and requires the use of live virus and cell culture.
SARS-CoV-2 virus is mixed with serum or plasma and incubated on a cell layer to induce infection in the cells. If antibodies are present in the serum/plasma, they will neutralise the virus and inhibit or prevent the infection of the cells. After incubation the cells are stained to visualise the presence of live virus. The test is time consuming, requiring 2-4 days to complete.
The SD Biosensor Standard F SARS-CoV-2 nAb test is a surrogate virus neutralisation test (sVNT) and is designed to mimic the virus-host interaction. The test detects total immuno-dominant neutralising antibodies (nAb) targeting the spike protein RBD in serum and plasma and uses recombinant ACE2 & RBD proteins instead of live cells & SARS-CoV-2 virus.
With the emergence of new variants of SARS-CoV-2, it is important to know if antibodies are providing immunity to more than one variant. This is particularly important as the South African and Brazil variants have a E484K mutation in the Receptor Binding Domain of S1.
The SD Biosensor Standard F SARS-CoV-2 nAb test includes 2 devices to differentiate neutralising antibodies to different variants within the assay time of 35 minutes. In a comparison to the traditional VNT the test shows 100% clinical sensitivity and 99% clinical specificity.

Potential applications:
- Retrospective contact tracing
- Seroprevalence surveying
- Assessment of herd immunity & longevity of protective immunity
- Assessment of vaccine efficacy
- Monitoring nAb titers in vaccinees after mass vaccination
SD Biosensor Standard F is a rapid point of care test system designed to be used in a laboratory or point of care facility by healthcare professionals.
For more information about Standard F or the SARS-CoV-2 nAb test please Contact Us.